|
|
Core Transcriptional Regulatory Circuitry in Human Embryonic Stem Cells
Acknowledgements References |
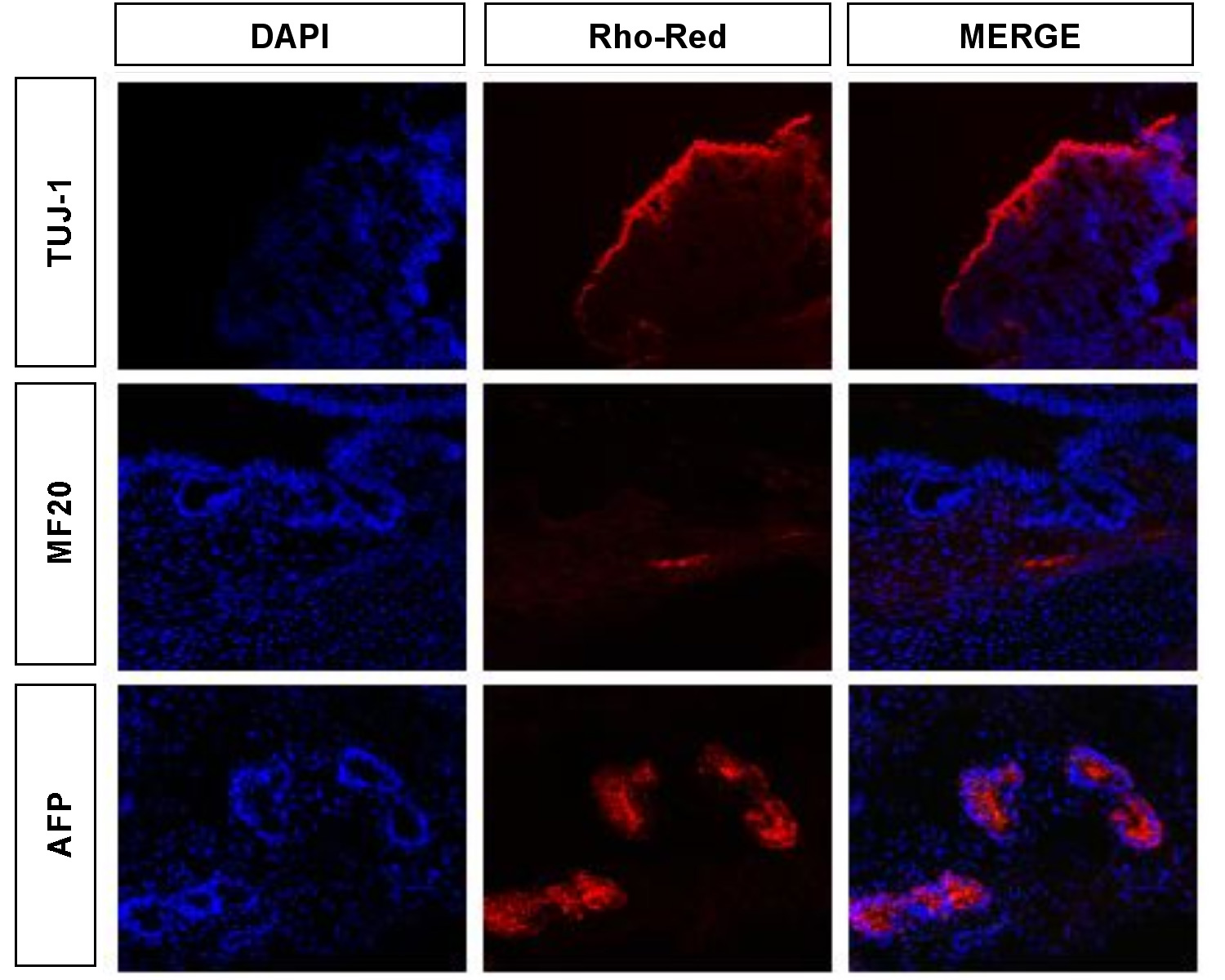
Teratomas were induced by injecting 2-5 x 10^6 cells into the subcutaneous tissue above the rear haunch of 6 week old Nude Swiss (athymic, immunocompromised) mice. Eight to twelve weeks post-injection, teratomas were harvested and fixed overnight in 4% paraformaldehyde at 4°C. Samples were then immersed in 30% sucrose overnight before embedding the tissue in O.C.T freezing compound (Tissue-Tek). Cryosections were obtained and 10µm sections were incubated with the appropriate antibodies as above and analyzed for the presence of the following differentiation markers by confocal microscopy (LSM 210): neuronal class II Beta-tubulin, Tuj1 (ectoderm; MMS-435P Covance); striated muscle-specific myosin, MF20 (mesoderm; kind gift from D. Fischman), and alphafetoprotein (endoderm; DAKO) (Figure S4). Nuclei were stained blue with 4’,6-diamidine-2-phenylidole dihydrochloride (DAPI). Antibody reactivity was detected for markers of all three germ layers confirming that the human embryonic cells used in our analysis have maintained differentiation potential.
Figure S4. Analysis of human ES cells for differentiation potential. Teratomas were analyzed for the presence of markers for ectoderm (Tuj1), mesoderm (MF20) and endoderm (AFP). For reference, nuclei are stained with DAPI. Antibody reactivity was detected for derivatives of all three germ layers confirming that the human embryonic cells used in our analysis have maintained differentiation potential. |
| YOUNG
LAB
Whitehead Institute 9 Cambridge Center Cambridge, MA 02142 [T] 617.258.5218 [F] 617.258.0376 CONTACT US |