|
|
Core Transcriptional Regulatory Circuitry in Human Embryonic Stem Cells
Acknowledgements References |
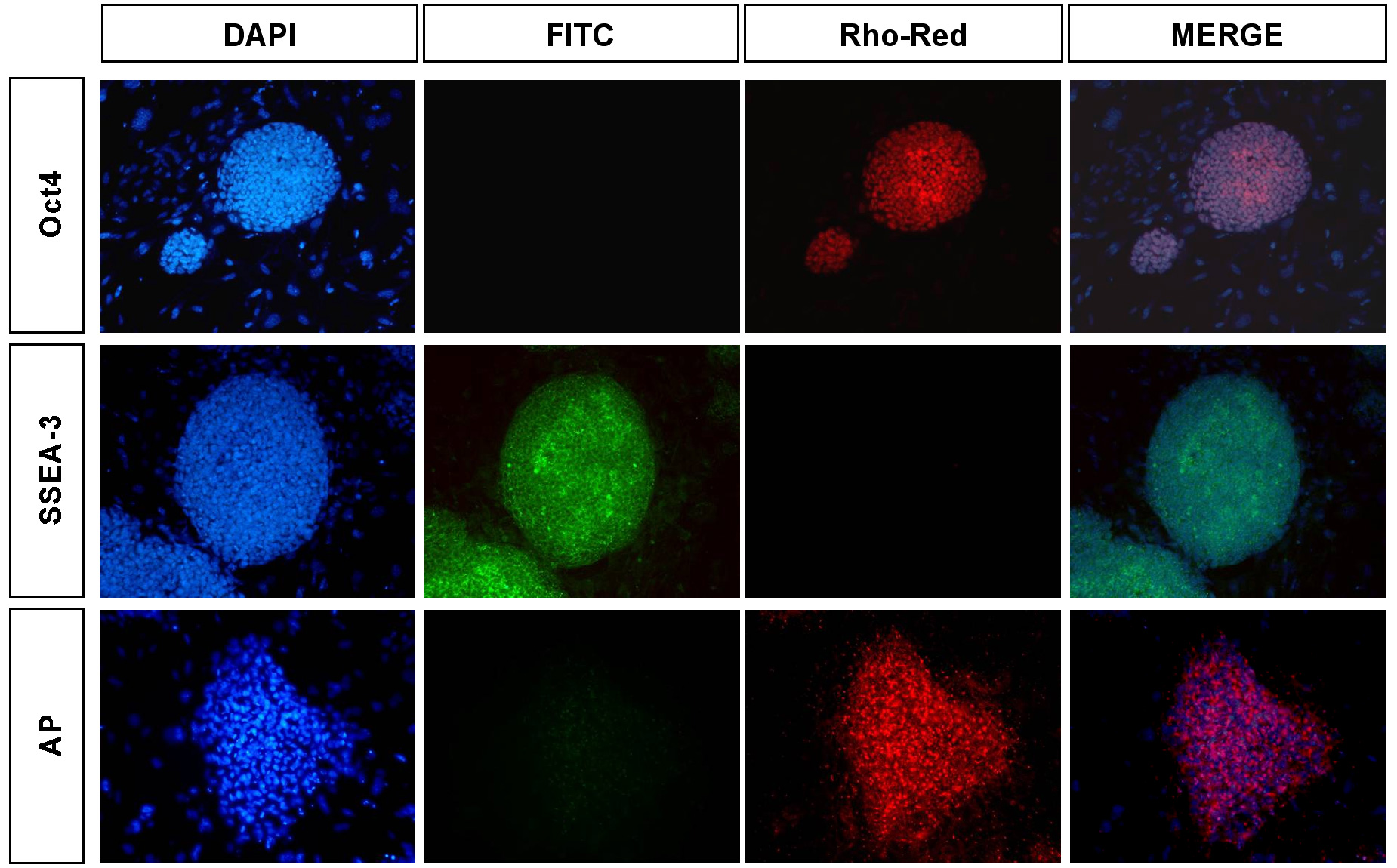
For analysis of pluripotency markers, cells were fixed in 4% paraformaldehyde for 30 minutes at room temperature and incubated overnight at 4°C in blocking solution (5 ml Normal Donkey Solution:195 ml PBS + 0.1% Triton-X)(Figure S3).
Figure S3. Analysis of human ES cells for markers of pluripotency. Human embryonic stem cells were analyzed by immunohistochemistry for the characteristic pluripotency markers Oct4 and SSEA-3. For reference, nuclei were stained with DAPI. Our analysis indicated that >80% of the cells were positive for Oct4 and SSEA-3. Alkaline phosphatase activity was also strongly detected in hESCs. After a brief wash in PBS, cells were then incubated with primary antibodies to Oct-3/4 (Santa Cruz sc-9081), SSEA-3 (MC-631; Solter and Knowles, 1979), SSEA-4 (MC-813-70; Solter and Knowles, 1979), Tra-1-60 (MAB4360; Chemicon International), and Tra-1-81 (MAB4381; Chemicon International) in blocking solution overnight at 4°C. Following incubation with primary antibody, cells were incubated with either rhodamine red or FITC-conjugated secondary antibody (Jackson Labs) for 2-5hrs at 4°C. Nuclei are stained with 4’,6-diamidine-2-phenylidole dihydrochloride (DAPI). Epifluorescent images were obtained using a fluorescent microscope (Nikon TE300). Data is shown for Oct4 and SSEA-3. Our analysis indicated that >80% of the H9 cells were strongly positive for all pluripotency markers. Alkaline phosphatase activity of human ES cells was analyzed using
the Vector Red Alkaline Phosphatase Subtrate Kit (Cat. No. SK-5100;
Vector Laboratories) according to manufacturer’s specifications
and the reaction product was visualized using fluorescent microscopy.
|
| YOUNG
LAB
Whitehead Institute 9 Cambridge Center Cambridge, MA 02142 [T] 617.258.5218 [F] 617.258.0376 CONTACT US |